Coulomb's law, developed in the 1780s by French physicist Charles Augustin de Coulomb, may be stated in scalar form as follows:
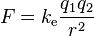
 The magnitude of the electrostatic force between two point electric charges is directly proportional to the product of the magnitudes of each charge and inversely proportional to the square of the distance between the charges.
The magnitude of the electrostatic force between two point electric charges is directly proportional to the product of the magnitudes of each charge and inversely proportional to the square of the distance between the charges.and in vector form as:

The magnitude of the force on a charge, q1, due to the presence of a second charge, q2, is given by the magnitude of where is the separation of the charges and is the electric constant. A positive force implies a repulsive interaction, while a negative force implies an attractive interaction.
Referring to Ionic Bond
 An ionic bond (or electrovalent bond) is a type of chemical bond that can often form between metal and non-metal ions (or polyatomic ions such as ammonium) through electrostatic attraction. In short, it is a bond formed by the attraction between two oppositely charged ions. The metal donates one or more electrons, forming a positively charged ion or cation with a stable electron configuration. These electrons then enter the non metal, causing it to form a negatively charged ion or anion which also has a stable electron configuration. The electrostatic attraction between the oppositely charged ions causes them to come together and form a bond.
An ionic bond (or electrovalent bond) is a type of chemical bond that can often form between metal and non-metal ions (or polyatomic ions such as ammonium) through electrostatic attraction. In short, it is a bond formed by the attraction between two oppositely charged ions. The metal donates one or more electrons, forming a positively charged ion or cation with a stable electron configuration. These electrons then enter the non metal, causing it to form a negatively charged ion or anion which also has a stable electron configuration. The electrostatic attraction between the oppositely charged ions causes them to come together and form a bond. Bond is the most valuable thing for us, it is simply like a brotherhood has. Naturally, bond is made when two people need each other, taking and giving. Nature has teached us about basic concept of bonding, of course yes we can refer to Coulomb's law. People and the electric charge have the same characteristic, each of both has a potency, called q. Some peoples may have negative potential, and the others have possitive one. Interaction occurs when there is k possiblity to contact each other, called uncertainty constant. We can't do anything about this constant, because it refers to will of God. When, the bond has been made, the important parameter is released, we called relationship distance r. the bond must be weak if the relationship distance is too far, by means that there is less frequency of comunication. We've already know that the strongest bonding in nature is ionic bond, the interaction between possitive and negative ions. So, why we don't make a better bond with our friends by sharing our possitive things, just like the Natrium has done to Chlor.
Bond is the most valuable thing for us, it is simply like a brotherhood has. Naturally, bond is made when two people need each other, taking and giving. Nature has teached us about basic concept of bonding, of course yes we can refer to Coulomb's law. People and the electric charge have the same characteristic, each of both has a potency, called q. Some peoples may have negative potential, and the others have possitive one. Interaction occurs when there is k possiblity to contact each other, called uncertainty constant. We can't do anything about this constant, because it refers to will of God. When, the bond has been made, the important parameter is released, we called relationship distance r. the bond must be weak if the relationship distance is too far, by means that there is less frequency of comunication. We've already know that the strongest bonding in nature is ionic bond, the interaction between possitive and negative ions. So, why we don't make a better bond with our friends by sharing our possitive things, just like the Natrium has done to Chlor.
No comments:
Post a Comment