
Molecules and particles will enter the cell via cell membrane. The size also determines how they can pass through the membrane. If the diameter of nanoparticles is big, they cannot enter passively, and only stay in interstitial fluid. Although the hyperthermia process will still go on, the effect is less effective than the nanoparticles stay in plasma cell. Since the transport via cell membrane becomes one of many considerations in nanomagnetic design, the review about cellular membrane transport is needed.



There are two principal mechanisms of outer cellular membrane transport. The transport mechanisms are passive transport and active transport. Passive transport permits the substance entering the cell via simple diffusion, facilitated diffusion and osmosis. Whereas, the active transport allows the particles pass via membrane by using energy to activate the protein gate.
Diffusion is the net passive movement of particles (atoms, ions or molecules) from a region in which they are in higher concentration to regions of lower concentration. It continues until the concentration of substances is uniform throughout. High Diffusion Rate depends on short distance, large surface area, big concentration difference (Fick’s Law). High temperatures will increase diffusion; large molecules will slow diffusion.
Diffusion is the net passive movement of particles (atoms, ions or molecules) from a region in which they are in higher concentration to regions of lower concentration. It continues until the concentration of substances is uniform throughout. High Diffusion Rate depends on short distance, large surface area, big concentration difference (Fick’s Law). High temperatures will increase diffusion; large molecules will slow diffusion.
Fick's first law relates the diffusive flux to the concentration field, by postulating that the flux goes from regions of high concentration to regions of low concentration, with a magnitude that is proportional to the concentration gradient (spatial derivative). In one (spatial) dimension, this is
where
- J is the diffusion flux in dimensions of [(amount of substance) length−2 time−1], example mol/(m^2)s. J measures the amount of substance that will flow through a small area during a small time interval.
- D is the diffusion coefficient or diffusivity in dimensions of [length2 time−1], example m^2/s.
 (for ideal mixtures) is the concentration in dimensions of [(amount of substance) length−3], example mol/m^3
(for ideal mixtures) is the concentration in dimensions of [(amount of substance) length−3], example mol/m^3
- x is the position [length], example m
D is proportional to the squared velocity of the diffusing particles, which depends on the temperature, viscosity of the fluid and the size of the particles according to the Stokes-Einstein relation. In dilute aqueous solutions the diffusion coefficients of most ions are similar and have values that at room temperature are in the range of 0.6x10−9 to 2x10−9 m2/s. For biological molecules the diffusion coefficients normally range from 10−11 to 10−10 m2/s.
In two or more dimensions we must use  , the del or gradient operator, which generalizes the first derivative, obtaining
, the del or gradient operator, which generalizes the first derivative, obtaining
 , the del or gradient operator, which generalizes the first derivative, obtaining
, the del or gradient operator, which generalizes the first derivative, obtaining
And for second law is:

Facilitated diffusion is the movement of specific molecules down a concentration gradient, passing through the membrane via a specific carrier protein. Thus, rather like enzymes, each carrier has its own shape and only allows one molecule (or one group of closely related molecules) to pass through. Selection is by size; shape; charge. Common molecules entering/leaving cells this way include glucose and amino-acids. It is passive and requires no energy from the cell. If the molecule is changed on entering the cell (glucose + ATP → glucose phosphate + ADP), then the concentration gradient of glucose will be kept high, and there will a steady one-way traffic.

Osmosis is a special example of diffusion. It is the diffusion of water through a partially permeable membrane from a more dilute solution to a more concentrated solution – down the water potential gradient (Note: diffusion and osmosis are both passive, i.e. energy from ATP is not used). A partially permeable membrane is a barrier that permits the passage of some substances but not others; it allows the passage of the solvent molecules but not some of the larger solute molecules. Cell membranes are described as selectively permeable because not only do they allow the passage of water but also allow the passage of certain solutes. The presence of particular solutes stimulates the membrane to open specific channels or trigger active transport mechanisms to allow the passage of those chemicals across the membrane.
Active transport is the energy-demanding transfer of a substance across a cell membrane against its concentration gradient, i.e., from lower concentration to higher concentration. Special proteins within the cell membrane act as specific protein ‘carriers’. The energy for active transport comes from ATP generated by respiration (in mitochondria). Major examples of active transport are re-absorption of glucose, amino acids and salts by the proximal convoluted tubule of the nephron in the kidney, sodium/potassium pump in cell membranes (especially nerve cells).
In order to make nanoparticles pass through cell membrane easily, some nanoparticles are designed by embedding with special protein, encapsulating with liposome, or just designed in very small sized (3-5 nm) to make them freely diffused.



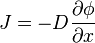


No comments:
Post a Comment